Using genome sequencing to predict treatment response in blood cancer
Myeloproliferative neoplasms (MPNs) are cancers that arise when a mutated blood stem cell begins to produce too many red blood cells, white blood cells, or platelets. A number of mutations can drive MPNs, and studies have demonstrated that different mutations result in different clinical outcomes. For example, between the two most commonly mutated genes, JAK2 and CALR, JAK2-mutated MPNs tend to be the more aggressive cancers.
Chemotherapy is currently the most widely used treatment for patients with MPNs, but an immunotherapy drug called IFNα has shown promise in recent years—some patients achieve remission even after treatment stops, which has not been observed with chemotherapy. But while mutation-specific disease outcomes are well-documented, the question remains: does a patient’s mutation affect their response to treatment?
To find out, former Damon Runyon Clinical Investigator Ann L. Mullally, MD, and her team at Dana-Farber Cancer Institute gathered tumor samples from MPN patients receiving either IFNα or chemotherapy. They sequenced the patient samples and measured how much the frequency of the mutant gene (primarily JAK2 or CALR) decreased after therapy. The greater the decrease, the more mutant tumor cells had been killed, and thus the more effective the treatment.
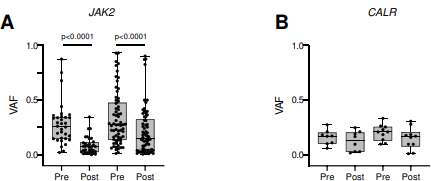
The team found that the response to IFNα was indeed mutation-specific. For patients with JAK2 mutations, IFNα induced a far greater response than did chemotherapy; for patients with CALR mutations, the two treatments were equally (and not very) effective. This accords with previous studies that have found CALR-mutant cells less sensitive to IFNα therapy than JAK2-mutant cells. Importantly, however, the team also found new mutations emerging after treatment—and this was more common in patients receiving IFNα.
Altogether, these findings highlight the importance of mutation-specific care in MPN, not only at diagnosis but in treatment counseling and post-treatment follow-up. With further research, Dr. Mullally and her lab aim to identify genetic markers that can better guide treatment decisions. As the clinical investigator recently tweeted: “Wouldn’t it be great to have low-cost sequencing methodologies that enable genomic profiling as a routine component of MPN clinical care?”
This research was published in Blood Advances.
