Toward a new understanding of cancer metabolism
Cancer cells are often assumed to be “hypermetabolic,” meaning their energy-producing cycles run on overdrive to fuel the uncontrolled division and growth that defines a tumor. But new findings from former Damon Runyon Fellow Caroline R. Bartman, PhD, and her colleagues at Princeton University challenge this assumption, revealing how much we still have to learn about cancer metabolism.
As you may recall from biology class, animal cells produce energy by converting sugar into ATP via the TCA cycle (perhaps better known as the Krebs cycle). To determine if cancer cells actually produce more energy than normal cells, Dr. Bartman and her team tracked and compared TCA cycle activity in healthy mouse tissue versus primary solid tumors, leukemia, and metastatic breast cancer tissue. Their approach, known as “isotope tracing,” involves infusing the tissue with a molecule that is functionally identical to the sugar being metabolized, but tagged so that it can be “traced” as it moves through the cell’s metabolic pathways.
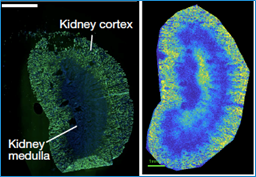
To their surprise, the team found that the rate of ATP synthesis was higher only in the leukemia and metastatic tissues; in all primary solid tumors, TCA cycle activity was lower than in healthy cells. Furthermore, in the mouse pancreatic tumor, the researchers noticed that this decrease in energy production was accompanied by reduced protein synthesis—one of the tissue’s major energy costs. In light of these observations, the team proposed a new model to explain how cancer cells proliferate uncontrollably without upping their energy production: they are energetically thrifty, scrapping expensive processes (like building proteins) in order to grow and divide on the cheap.
This updated understanding of the developing tumor’s energy usage may give rise to new therapeutic strategies—if, given their low rates of ATP synthesis, cancer cells are more vulnerable to ATP deprivation than previously thought. Targeted therapies that enhance the ATP-dissolving enzyme ATPase, for example, may prove effective in slowing tumor growth.
This research was published in Nature.
