New lymphoma drug shows promise in clinical trials

Follicular lymphoma is a slow-growing cancer that occurs when the body produces abnormal B cells that form clumps, or “follicles,” in the lymph nodes. Like T cells, B cells are a type of white blood cell integral to the immune system. Unlike T cells, which attack the body’s own cells when they become infected or cancerous, B cells produce antibodies that target invading bacteria, viruses, and other pathogens.
Because follicular lymphoma develops slowly, it can usually be treated before it reaches an advanced stage. Unfortunately, however, about one in five patients experiences relapse within two years of starting treatment, and the lymphoma comes back more aggressive. At this point, treatment options are scarce.
But a recent clinical trial led by former Damon Runyon Clinical Investigator L. Elizabeth Budde, MD, PhD, at City of Hope National Medical Center offers hope for these patients. In the study, 80 percent of participants with relapsed follicular lymphoma responded to a new drug called mosunetuzumab, with 60 percent experiencing complete remission. This is a significantly higher response rate than has been achieved by other treatments, and represents an exciting step forward.
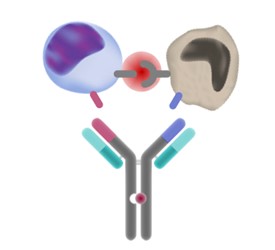
Mosunetuzumab is what is called a bispecific antibody, meaning it binds to two targets simultaneously. One of these targets resides on the surface of T cells, where it serves as a kind of “on” button, and the other is expressed by malignant B cells. By binding both targets at once, the drug recruits T cells to attack cancerous B cells.
Given its promise thus far in clinical trials, the team is hopeful that larger-scale studies of mosunetuzumab will confirm its benefit for patients with relapsed lymphoma and eventually lead to its approval for use in the clinic.
This study was published in The Lancet Oncology.
